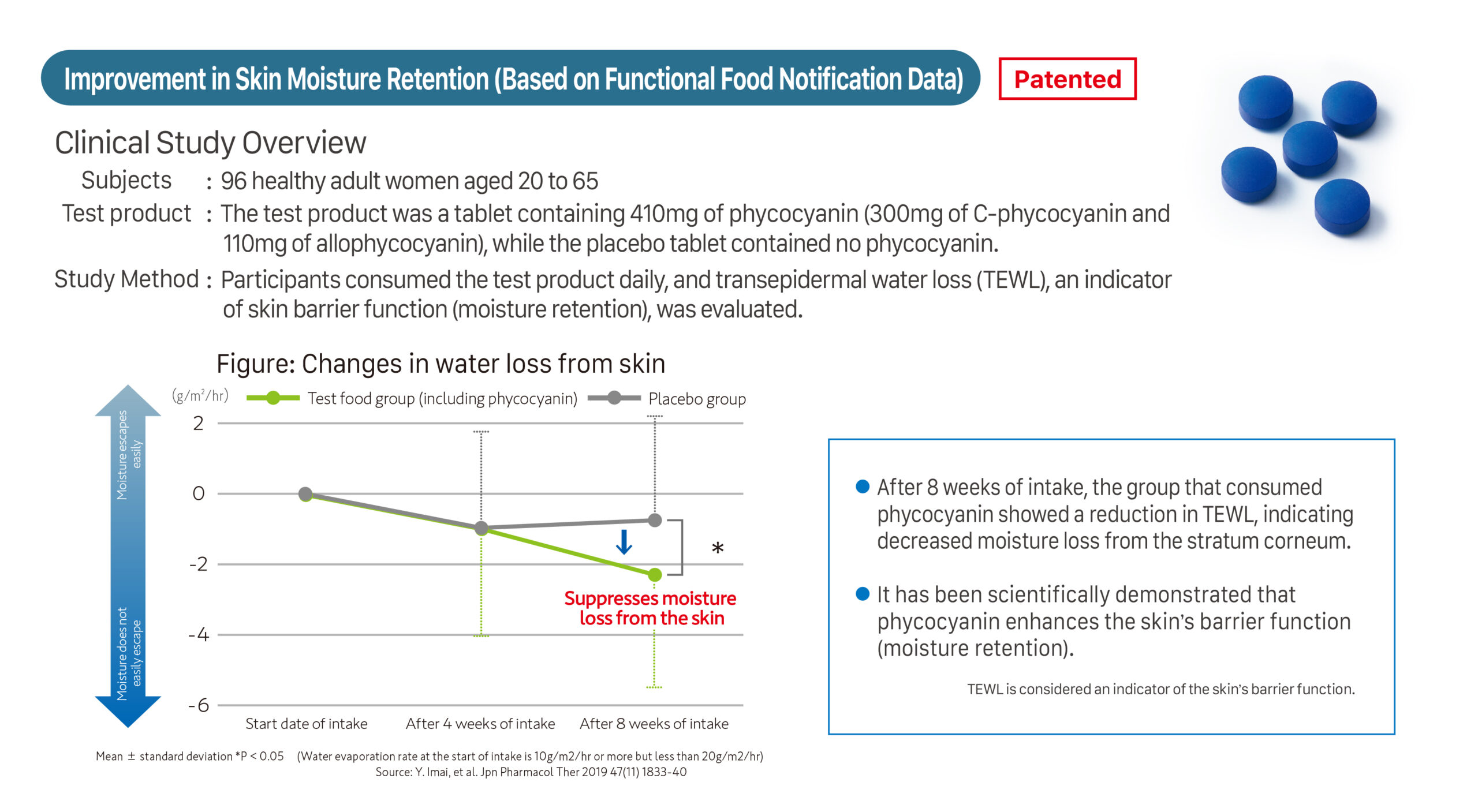
Subjects: 93 healthy adult women aged 20 to 62
Test product: Tablet containing phycocyanin (410mg of phycocyanin: 300mg of C-phycocyanin and 110mg of allophycocyanin)
Study Method: Participants consumed the test product daily, and changes in VAS (Visual Analog Scale) scores from baseline were evaluated. (**P < 0.01, *P < 0.05)
Commercial Products For Food / Dietary Supplement Phycocyanin Powder
Phycocyanin Powder is a product containing drinkable skincare ingredient, "phycocyanin", derived from the blue-green algae spirulina. Phycocyanin Powder contains "C-phycocyanin" and "allophycocyanin", structurally distinct components, in an optimal ratio to be effective. Recently, we have confirmed by clinical studies its skincare benefits including improved skin barrier function (moisture retention), wrinkle reduction, enhanced firmness and radiance through oral intake.
●Features of Phycocyanin Powder
① Proven by a clinical study. Enhances Skin Barrier Function (Moisture Retention) and Hydration.

② Improvement in skin moisture, firmness, and radiance
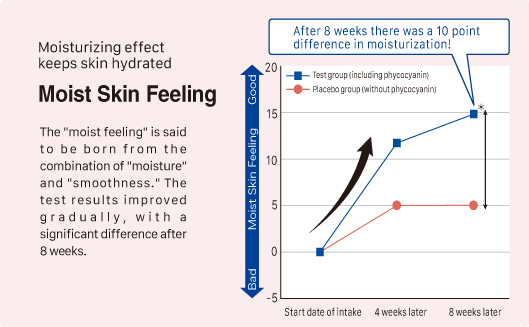
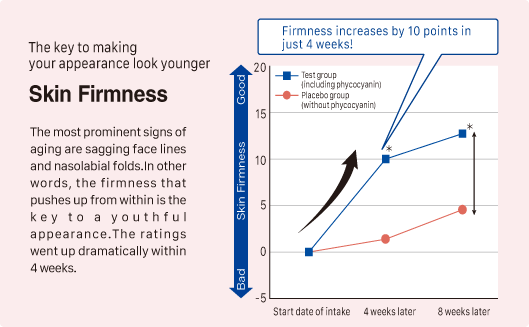
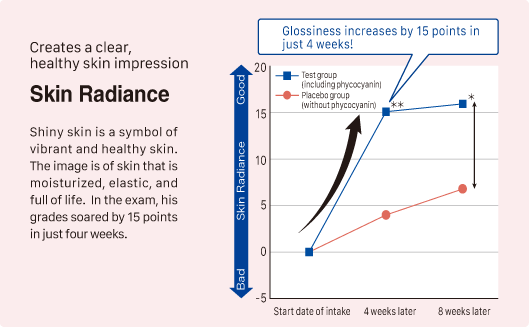
Source: Y. Imai, et al. Jpn Pharmacol Ther 2019 47 (11) 1833-40
③ Improvement in wrinkles
Subjects: 93 healthy adult women aged 20 to 62
Test Product: Tablet containing phycocyanin (410mg of phycocyanin: 300mg of C-phycocyanin and 110mg of allophycocyanin)
Study Method: Participants consumed the test product daily, and the condition of their skin wrinkles was evaluated using the replica method. (*P < 0.05)
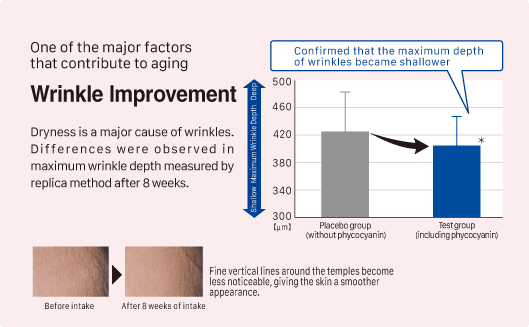
Source: Y. Imai, et al. Jpn Pharmacol Ther 2019 47 (11) 1833-40
④ High Quality InーHouse production
DIC Corporation achieved the world’s first mass production of Spirulina under controlled cultivation in 1977, and has since been committed to delivering safe and high-quality Spirulina products. Phycocyanin Powder uses a proprietary extraction and purification process to obtain phycocyanin from premium-quality Spirulina.
Use Patent: Japanese Patent No. 6784947 Foods and beverages for healthy skin
●Safety
1. Acute Toxicity Test: The LD50value in both male and female mice is over 30,000 mg/kg
2. Chronic Toxicity Test: No chronic toxicity factors were detected in either male or female mice in a 12-month chronic toxicity study using a feed containing 1% of the test substance . Additionally, the test results were negative for carcinogenicity in all organs.
※Regarding spirulina as the raw material, safety has been confirmed through subacute toxicity tests, chronic toxicity tests, and multigenerational studies.
●Quality Control
Certifications and Standards: Our operations are certified with HALAL and KOSHER. Also it adopts globally recognized quality management systems including FSSC 22000, ISO 9001, and HACCP to ensure the highest level of safety and quality.



 Product Search
Product Search Request Documents
Request Documents
 Online store
Online store